 Senin, 20 Januari 2025 (17:39)
Senin, 20 Januari 2025 (17:39)
 Music |
 Video |
 Movies |
 Chart |
 Show |
 |
8.49 | Explain why N2 2+ is diamagnetic, while O2 4+, which has the same number of valence electrons (The Glaser Tutoring Company) View |
 |
Why is O2 paramagnetic (OneClass) View |
 |
Why N2 is diamagnetic and O2 is paramagnetic| Similarities between VBT and MOT| Chemistry Sciences (Chemistry Sciences) View |
 |
Molecular Orbital (MO) Diagram for O2(-) (chemistNATE) View |
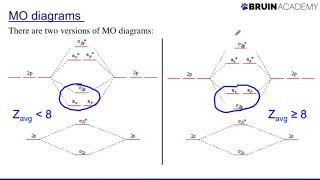 |
Drawing Molecular Orbital Diagrams (Bruin Academy) View |
 |
Quantum Numbers, Atomic Orbitals, and Electron Configurations (Professor Dave Explains) View |
 |
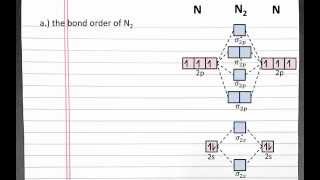
Molecular Orbital Theory. C2, N2, O2 and F2 molecules (Diego Troya) View |
 |
CHEMISTRY 101: Molecular Orbital Theory, Bond order, bond strength, magnetic properties (Matthew Gerner) View |
 |
CHEM 101: Applying Molecular Orbital Theory (Matthew Gerner) View |
 |
8.35 | Can a molecule with an odd number of electrons ever be diamagnetic Explain why or why not. (The Glaser Tutoring Company) View |